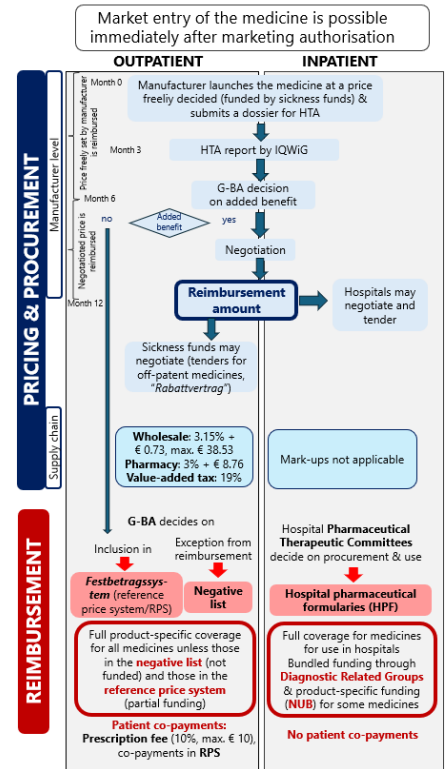
The 2025 edition of PPRI Pharma Brief on Germany provides a comprehensive and up-to-date overview of the German pharmaceutical system, highlighting recent policy developments. This edition is complemented by a detailed flowchart and a list of key stakeholders.
PPRI Pharma Briefa are concise reports that summarise the landscape of pharmaceutical pricing, procurement and reimbursement policy landscape in a country. They are part of a series of country-specific publications compiled by experts within the Pharmaceutical Pricing and Reimbursement Information (PPRI) network.
Link to the PPRI Pharma Brief on Germany: https://ppri.goeg.at/system/files/inline-files/PPRI_Pharma_Brief_DE_2025_bf.pdf
Please cite as: Vogler S. PPRI Pharma Brief: Germany 2025. Pharmaceutical Pricing and Reimbursement Information (PPRI) Pharma Briefs Series. Vienna: Gesundheit Österreich (GÖG / Austrian National Public Health Institute), 2025
© Graph by author (PPRI Secretariat)
